Understanding Chemical Bonding (Ionic vs. Covalent): A Beginner's Guide
Have you ever wondered what holds the world together? Why the salt on your table is a tiny crystal, while the water in your glass is a liquid? The answer to these questions, and countless others in science, lies in one of the most fundamental concepts in chemistry: chemical bonding.
At its simplest, a chemical bond is an attraction between atoms that allows them to form molecules and compounds. It’s the "glue" of the universe.
For many students, this topic can feel abstract and confusing. But it doesn’t have to be. The two most common types of chemical bonds—ionic and covalent—are driven by one simple goal that all atoms share. Once you understand that goal, everything else clicks into place. This guide will use simple analogies to demystify these crucial concepts and build you a rock-solid foundation in chemistry.
The "Why" of Bonding: The Octet Rule
Before we look at how atoms bond, we need to understand why they do it. The reason is stability.
Atoms “want” to be stable, and the most stable atoms in the universe are the noble gases (like Neon, Argon, and Krypton). What makes them so special? They have a full outer shell of electrons. For most elements, this means having eight valence electrons, a principle known as the octet rule.
Think of it like a game of musical chairs where every atom wants to have a full set of 8 chairs. Atoms that don’t have a full outer shell are unstable and reactive. They will eagerly interact with other atoms—by giving, taking, or sharing electrons—to achieve that stable, noble gas configuration. This interaction is what creates a chemical bond.
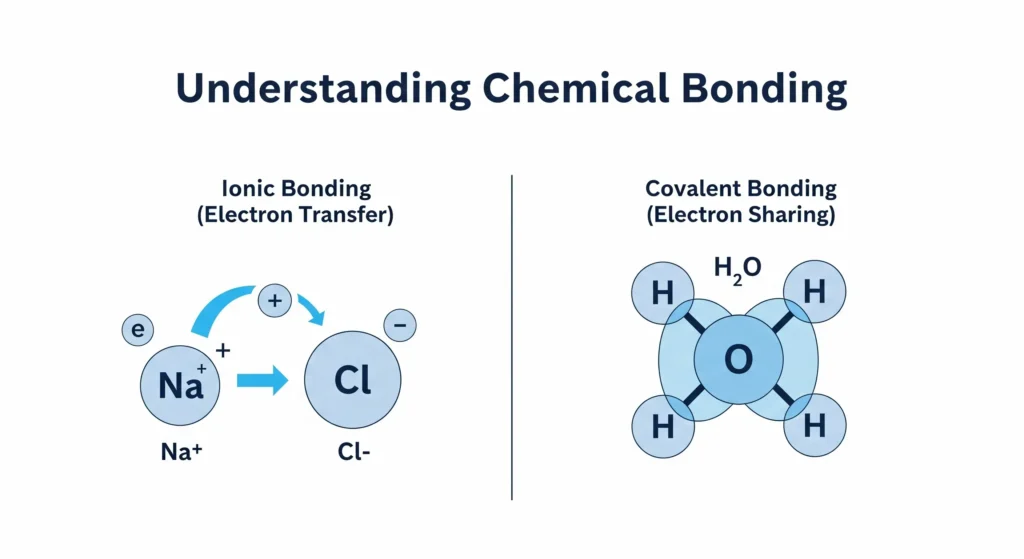
Ionic Bonding: The Great Electron Giveaway
The first type of bond, the ionic bond, is all about the transfer of electrons.
Analogy: Imagine you have a friend who is a picky eater and has one extra sandwich they don’t want (a metal). You have another friend who is very hungry and needs just one more sandwich to feel full (a non-metal). The first friend generously gives their sandwich to the hungry friend. Now, everyone is happy and stable.
That’s exactly how ionic bonding works. It typically happens between a metal and a non-metal.
The Giver (Metal): Metals, like Sodium (Na) or Magnesium (Mg), have only one or two electrons in their outer shell. It’s much easier for them to lose these few electrons to achieve a full shell underneath.
The Taker (Non-metal): Non-metals, like Chlorine (Cl) or Oxygen (O), have six or seven electrons in their outer shell. It’s much easier for them to gain one or two electrons to complete their octet.
When the metal atom transfers its electron(s) to the non-metal, they are no longer neutral atoms. They become charged particles called ions.
The metal atom, having lost a negative electron, becomes a positively charged ion (cation).
The non-metal atom, having gained a negative electron, becomes a negatively charged ion (anion).
The ionic bond is the powerful electrostatic force of attraction between these oppositely charged ions. It’s like the powerful attraction between the north and south poles of two magnets.
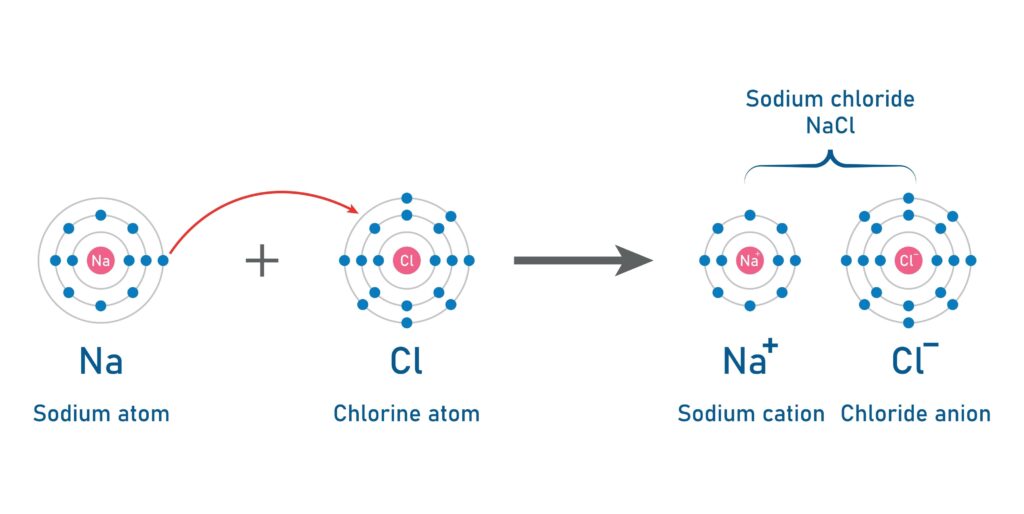
Classic Example: Sodium Chloride (NaCl) - Table Salt
A checklist breaks down a big topic into manageable chunks. Here’s a sample for “Alcohols”:
Sodium (Na) has 1 valence electron. Chlorine (Cl) has 7. Sodium transfers its one electron to Chlorine. Sodium becomes Na⁺, and Chlorine becomes Cl⁻. The powerful attraction between Na⁺ and Cl⁻ forms a strong ionic bond.
The formation of ions and the concept of electrostatic attraction can be a tricky concept. It’s a foundational idea in chemistry, and making sure you get this right with the guidance of a specialist chemistry tutor is key to building a strong understanding for more advanced topics.
Covalent Bonding: The Cooperative Share
The practical component is not just about getting the right results; it’s about demonstrating competency.
What happens when two atoms are both “takers”? What if two hungry friends both want another sandwich, and neither is willing to give one up? They might decide to pool their resources and share.
This is the basis of covalent bonding. A covalent bond is all about the sharing of electrons. It typically happens between two non-metal atoms.
The Situation: Two non-metals both need to gain electrons to achieve a stable octet.
The Solution: Instead of transferring, they each contribute one or more electrons to form a shared pair.
The Bond: This shared pair of electrons is attracted to the positive nuclei of both atoms, holding them together in a strong bond.
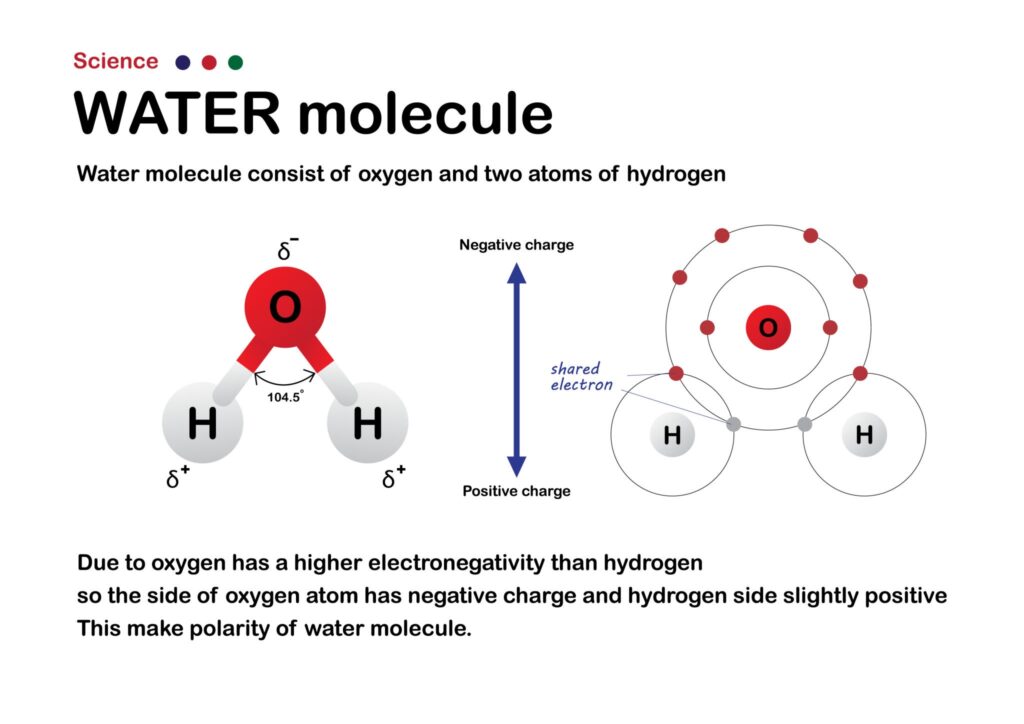
Classic Example: Water (H₂O) Oxygen needs two more electrons to get a full shell of eight. Each Hydrogen atom needs one more electron to get a full shell of two. The Oxygen atom shares one electron with one Hydrogen, and a second electron with another Hydrogen. Everyone is now stable and happy.
Single, Double, and Triple Bonds
Atoms can share more than one pair of electrons:
Single Bond: One shared pair (e.g., in H-H or Cl-Cl).
Double Bond: Two shared pairs (e.g., in O=O).
Triple Bond: Three shared pairs (e.g., in N≡N), which is an incredibly strong bond.
Visualizing how these shared pairs create 3D molecular shapes, like the famous “bent” shape of water, is a crucial skill in chemistry. This is another area where the abstract concept can be made clear with personalized attention, as an experienced chemistry tutor in Dubai can help you visualize these structures with models and simple drawing techniques.
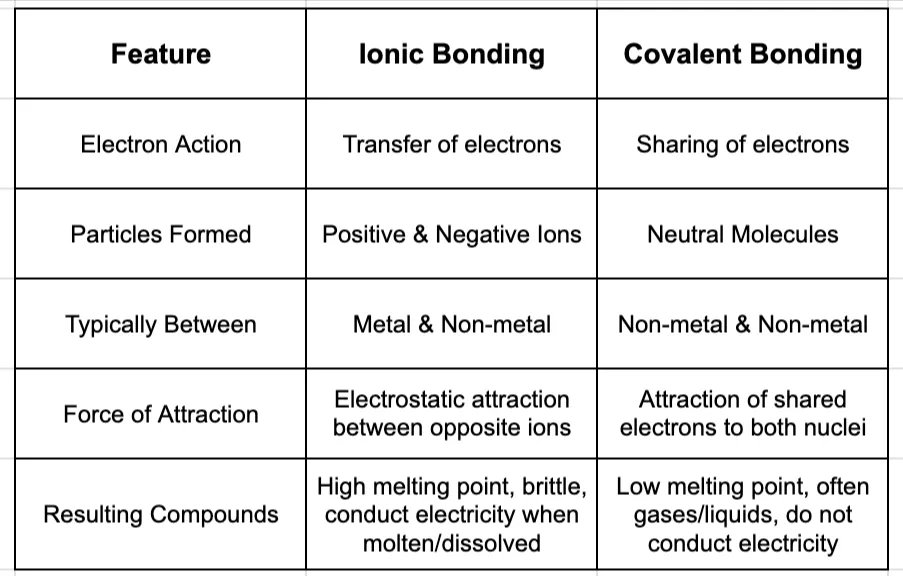
Summary: Ionic vs. Covalent at a Glance
Conclusion: The Foundation of Everything
Understanding the fundamental difference between ionic (transfer) and covalent (share) bonding is one of the most important steps you will take in your chemistry education. This single concept explains the properties of millions of different compounds and lays the groundwork for understanding chemical reactions.
The key is to remember the “why”—that universal drive for atoms to achieve a stable, full outer shell of electrons. Whether by giving, taking, or sharing, bonding is the process that builds our world.
If you are ready to build a rock-solid foundation in chemistry and want to ensure you have the expert support to master this and other key topics, learn more about how our specialist tutors can help you achieve your goals.