Organic Chemistry Basics: Alkanes, Alkenes & Alkynes Explaine
For many chemistry students, the moment the teacher writes "Organic Chemistry" on the board is a moment of dread. It has a reputation for being incredibly complex, a subject filled with endless reactions and impossible-to-remember names.
But what if we told you that the entire, vast world of organic chemistry is built on a few simple, logical rules? What if you could understand the fundamentals so well that the rest of the subject clicks into place like a puzzle?
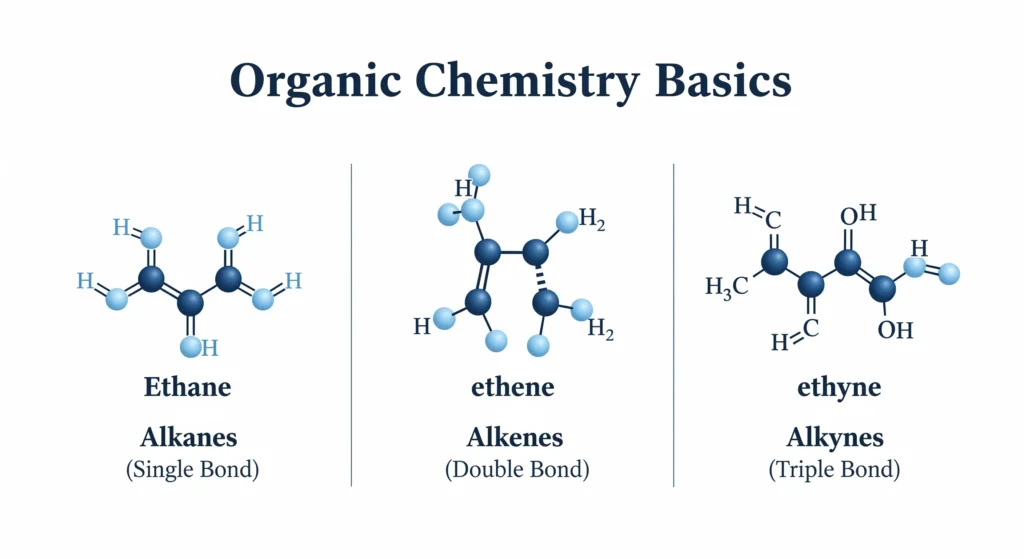
That’s the goal of this guide. We are going to demystify the absolute basics. Forget about complex reactions for a moment. We’re going back to the beginning to build a rock-solid foundation by understanding the three most fundamental families of organic compounds: the alkanes, alkenes, and alkynes.
What is Organic Chemistry? The Chemistry of Carbon
At its heart, organic chemistry is the study of carbon-containing compounds. Carbon is a special element because it can form four strong covalent bonds, allowing it to link together in long chains and complex rings, creating the millions of molecules that form the basis of life itself—from DNA to the food we eat.
The simplest organic molecules are called hydrocarbons, which, as the name suggests, contain only carbon and hydrogen atoms. These are the building blocks we will focus on today.
The First Family: Alkanes (The Saturated Hydrocarbons)
Think of alkanes as the stable, predictable foundation of organic chemistry.
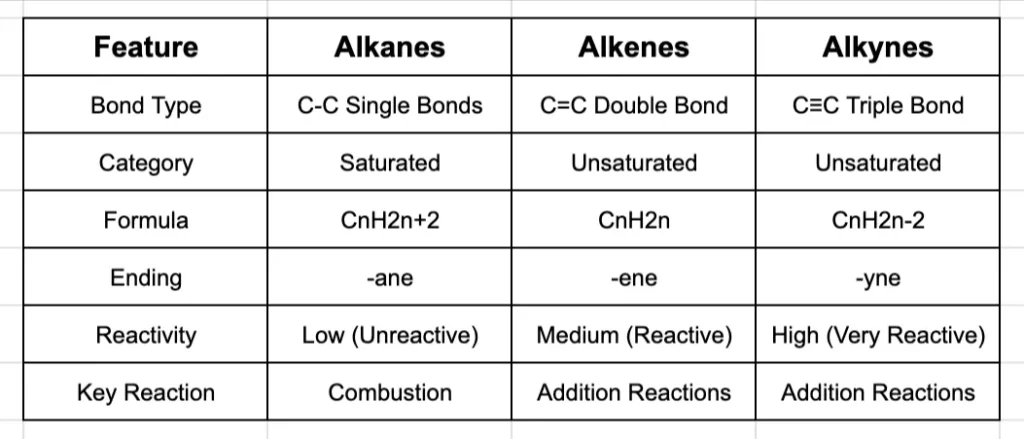
Definition: Alkanes are hydrocarbons where all the carbon-to-carbon bonds are single bonds.
Key Feature: They are known as saturated hydrocarbons. This is a crucial term. It means each carbon atom is bonded to the maximum possible number of other atoms (four). There’s no room for any more atoms to be added.
General Formula: The number of hydrogen atoms in any alkane can be found using the formula CnH2n+2, where ‘n’ is the number of carbon atoms.
Naming Alkanes: The names of the first four alkanes are historical, but from five carbons onwards, it follows a simple Greek prefix system. You must memorize the first ten.
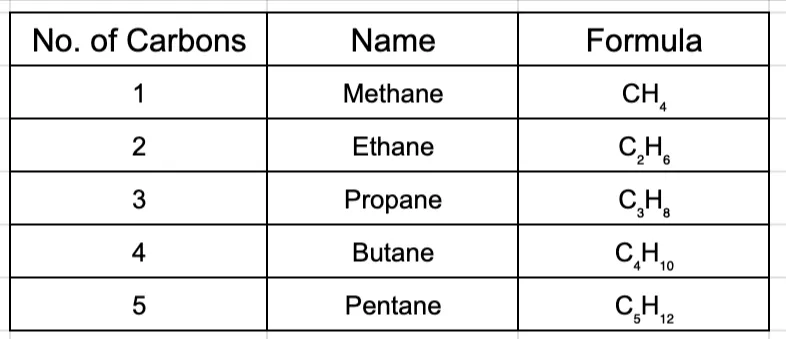
Notice how they all end in -ane. This is the suffix for all alkanes.
Properties of Alkanes:
Because all the bonds are single covalent bonds (C-C and C-H), they are relatively strong and stable. This makes alkanes quite unreactive. Their main reaction is combustion (burning in oxygen), which is why they make excellent fuels like natural gas (methane) and petrol.
The Second Family: Alkenes (Introducing Double Bonds)
If alkanes are the stable foundation, alkenes are where things start to get interesting.
Definition: Alkenes are hydrocarbons that contain at least one carbon-to-carbon double bond (C=C).
Key Feature: They are unsaturated. The presence of the double bond means the carbon atoms involved are not bonded to the maximum number of other atoms. The double bond can “open up” to allow new atoms to be added.
General Formula: For alkenes with one double bond, the formula is CnH2n.
Naming Alkenes: The naming follows the same prefixes, but the ending is changed to -ene.
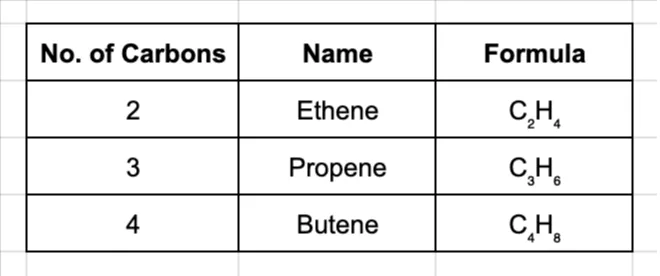
For butene and larger molecules, you need to specify where the double bond is. For example, but-1-ene has the double bond after the first carbon, while but-2-ene has it after the second.
Reactivity of Alkenes: That C=C double bond is a region of high electron density, making alkenes much more reactive than alkanes. Their signature reaction is the addition reaction, where the double bond breaks and new atoms are added across it. For example, ethene can react with hydrogen (hydrogenation) to become ethane.
Understanding how these reactions work often involves visualizing the movement of electrons, which can be tricky at first. It’s a common area where students get stuck. If you find the concept of electrophilic addition challenging, know that working through it step-by-step with a specialist organic chemistry tutor can often make the mechanism click into place.
The Third Family: Alkynes (Triple Bonds)
Alkynes are even more reactive and unsaturated than alkenes.
Definition: Alkynes are hydrocarbons that contain at least one carbon-to-carbon triple bond (C≡C).
Key Feature: They are also unsaturated, and even more so than alkenes.
General Formula: For alkynes with one triple bond, the formula is CnH2n-2.
Naming Alkynes:
The naming follows the same prefixes, but the ending is changed to -yne.
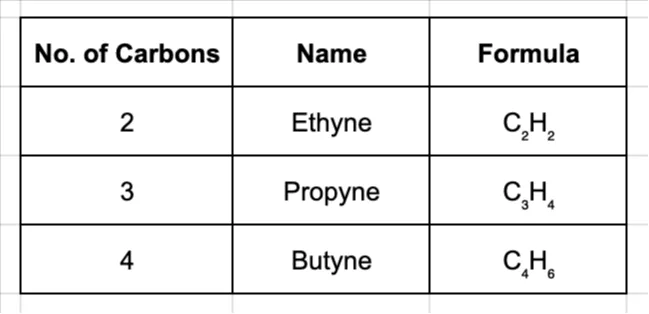
Reactivity of Alkynes:
The triple bond is a region of very high electron density, making alkynes extremely reactive. Like alkenes, they undergo addition reactions, but because they have two weak pi bonds (in the triple bond), they can often react twice.
Summary: A Clear Comparison
The Key to Success: Think in 3D
The biggest mistake students make in organic chemistry is trying to memorize everything as flat drawings on a page. These molecules are 3D objects. Start practicing drawing them. Use molecular modeling kits if you have them. Understanding the shape of a molecule is the first step to understanding its reactivity. For many students, this visualization is the hardest part. Sometimes, all it takes is having someone sit down with you and explain the shapes and reaction mechanisms one-on-one. The support of an experienced chemistry tutor in Dubai can be invaluable in building this foundational skill.
Conclusion: Your Journey Starts Here
You now have a solid understanding of the three fundamental families that form the basis of organic chemistry. You know the difference between saturated and unsaturated, you can name the basic compounds, and you understand why their reactivity differs.
This is your foundation. Every other organic compound you will study—alcohols, carboxylic acids, esters—is simply one of these hydrocarbon chains with a different functional group attached. Master these basics, and you will be well on your way to mastering the entire subject.
If you are ready to build on this foundation and want to ensure you have the expert support needed to excel in your studies, learn more about how our specialist tutors can help you achieve your goals.