How to Balance Chemical Equations: The Easy Way

Welcome to one of the most fundamental skills in all of chemistry: balancing chemical equations. If you’re just starting out, the idea of balancing equations might seem like a complex puzzle. But the truth is, it’s more like following a simple recipe. Once you learn the rules and the step-by-step process, it’s a skill you’ll use with confidence throughout your chemistry studies.
This guide will demystify the entire process. We’re going to break it down into simple, repeatable steps and walk through clear examples, turning this core skill from a point of confusion into a point of confidence.
Why Do We Need to Balance Equations? The Golden Rule
Before we get to the “how,” let’s understand the “why.” The entire reason we balance chemical equations is to satisfy one of the most important laws in all of science: The Law of Conservation of Mass.
This law states that in a chemical reaction, matter is not created or destroyed.
Think of it like building with LEGOs. If you start with 10 red bricks and 15 blue bricks, you can build whatever you want, but at the end, you must still have 10 red bricks and 15 blue bricks in your final creation. You can’t magically end up with 11 red bricks or have a blue one vanish.
A chemical equation is the same. The atoms you start with on the left side (the reactants) must be fully accounted for on the right side (the products). Balancing the equation is simply our way of doing that accounting.
The Method: Balancing by Inspection
The most common way to balance equations is “by inspection,” which is a fancy way of saying we’ll adjust the numbers until it works. We do this by placing large numbers, called coefficients, in front of the chemical formulas.
The #1 Most Important Rule: NEVER, EVER change the small subscript numbers within a chemical formula. Changing a subscript changes the actual substance. For example, H₂O is water. If you change the subscript to H₂O₂, you’ve made hydrogen peroxide—a completely different chemical! We can only change the amount of each substance by using coefficients.
Notice how they all end in -ane. This is the suffix for all alkanes.
The 4-Step Process to Balancing Any Equation
Here is a simple, repeatable method that works for most equations you’ll encounter at the IGCSE, A-Level, or AP level.
Step 1: Create an Inventory. Draw a simple table and list all the elements present in the reaction. Count the number of atoms for each element on both the reactants side (left) and the products side (right).
Step 2: Start with the “Ugliest” Molecule. Look for the most complex-looking compound in the equation (the one with the most elements or the most atoms). Begin by placing coefficients to balance the elements in that compound first. It’s often best to leave single elements (like O₂ or Fe) until the very end.
Step 3: Balance One Element at a Time. Place a coefficient in front of a molecule to balance one element. When you do this, immediately update your inventory table to reflect the new atom count for all elements in that compound. This is the key step. Go back and forth, adjusting coefficients and updating your inventory until all elements are balanced.
Step 4: Final Check. Once your inventory shows the same number of atoms for every element on both sides, do a final check. Make sure your coefficients are in the lowest possible whole-number ratio. For example, if you have 4, 2, 4, you can simplify that to 2, 1, 2.
Worked Example 1: The Combustion of Methane
Let’s balance the equation for burning natural gas (methane): CH₄ + O₂ → CO₂ + H₂O
Step 1: Create an Inventory.
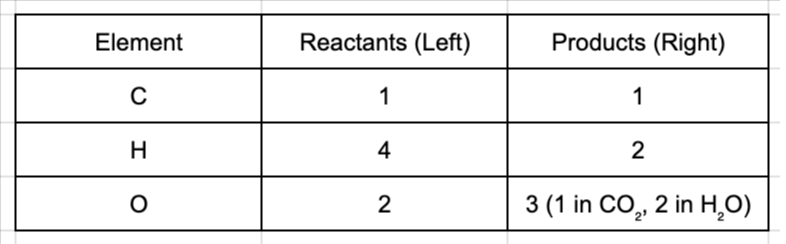
The carbons are balanced, but the hydrogens and oxygens are not.
Step 2 & 3: Balance One Element at a Time.
Let’s start with Hydrogen (H). We have 4 on the left and 2 on the right. To get 4 on the right, we need to place a coefficient of 2 in front of H₂O.
CH₄ + O₂ → CO₂ + 2H₂O
Now, let’s update our inventory. This change also affects oxygen!
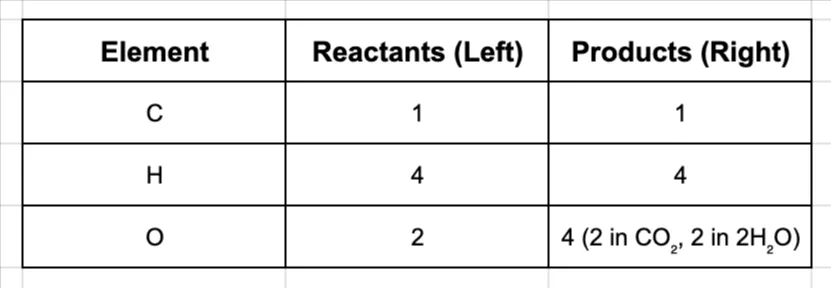
Step 4: Final Check
Everything is balanced! The coefficients are 1, 2, 1, 2, which is the lowest whole-number ratio.
The balanced equation is: CH₄ + 2O₂ → CO₂ + 2H₂O
While the process is logical, it’s easy to get lost in the numbers, especially when you’re just starting. If you find yourself getting stuck in a loop or feeling overwhelmed, remember that this is a common challenge. Getting personalized guidance from a dedicated chemistry tutor can often provide the clarity needed to master this essential skill.
From Struggle to Strength: A Student's Journey

Sameera K. , Former IGCSE Student, Dubai
When I was a student doing IGCSE chemistry, I initially struggled with understanding how chemical reactions actually worked beyond just memorizing equations. One of the most confusing topics for me was balancing chemical equations and figuring out why reactions happen the way they do.
Later on, my teacher explained it using simple real-life examples such as comparing atoms to people at a party who need to pair up correctly, or showing us experiments in the lab where we could actually see reactions taking place. That completely changed the way I looked at chemistry. Suddenly, it wasn’t just numbers and symbols on paper, but a real process happening around us every day.
Over time, I started to enjoy topics like acids and bases, rates of reaction, and organic chemistry. What once felt like the hardest part of my IGCSEs ended up becoming one of my strongest subjects. The experience taught me not just chemistry, but also patience and persistence in problem-solving.
Helpful Tips for Trickier Equations
Treat Polyatomic Ions as a Single Unit: If an ion like sulfate (SO₄²⁻) or phosphate (PO₄³⁻) appears unchanged on both sides of the equation, you can balance it as a single “chunk” instead of balancing the individual S and O atoms.
Balance H and O Last: Hydrogen and oxygen often appear in multiple compounds, making them harder to balance. It’s usually easiest to save them for the end.
The “Odd/Even” Trick: If you have an odd number of an element on one side and an even number on the other (often happens with O₂), a good trick is to double all the coefficients you have placed so far and then try again. This will make all your numbers even and easier to balance.
Many students find that applying these tricks to more complex problems is where the real challenge lies. When you’re facing a complicated equation and aren’t sure where to start, having an experienced chemistry tutor in Dubai to walk you through the strategy can be incredibly helpful.
Conclusion: From Puzzle to Process
Balancing chemical equations is a fundamental skill that transforms a qualitative statement into a precise, quantitative recipe. It’s a puzzle that, at first, might seem confusing, but by following a consistent, step-by-step process, you can solve it every single time.
Master this skill through practice. Write out your inventory, be methodical, and double-check your work. With every equation you balance, you are not just preparing for an exam; you are learning to speak the universal language of chemistry.
If you are committed to building a strong foundation and want to ensure you have the expert support to master this and other key topics, learn more about how our specialist tutors can help you achieve your goals.