A Complete Guide to Atomic Structure and the Periodic Table for AQA GCSE Combined Science Students in Dubai
Welcome to your ultimate guide for mastering one of the most fundamental topics in your AQA GCSE Combined Science course: Atomic Structure and the Periodic Table. From the gleaming aluminium that clads the Burj Khalifa to the air you breathe, everything in our world is built from tiny particles called atoms. Understanding them is the key to unlocking the secrets of chemistry.
This guide is specifically designed for you—a student in Dubai studying the AQA GCSE Combined Science: Trilogy (8464) specification. We will cover every key concept from Topic 1 (Chemistry Paper 1) in exactly the right depth, ensuring you are perfectly prepared for your exams. We’ll break down complex ideas into simple, manageable steps, use examples you can see around you every day in Dubai, and build your confidence from the ground up.
Let’s dive in and explore the building blocks of our universe.
Contents
The Building Blocks of Everything: The Atom
For thousands of years, scientists have developed and refined their ideas about what matter is made of. The model we use at GCSE is a powerful one: it describes the atom as having a tiny, dense, positively charged nucleus at its centre, with negatively charged electrons orbiting it in specific energy levels, or shells.
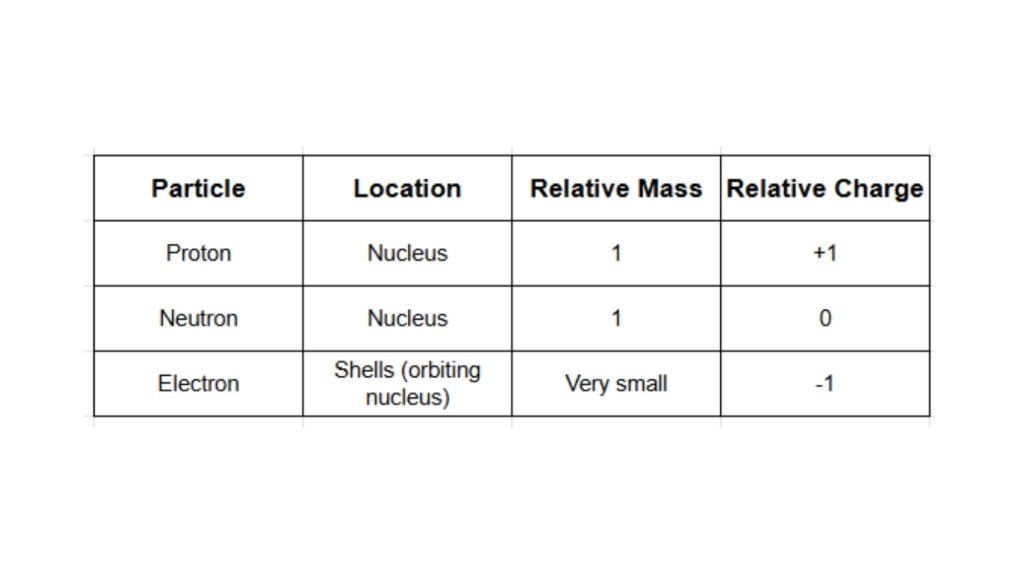
The nucleus itself is made of two types of particles, while a third type orbits outside. These are the three fundamental subatomic particles you need to know.
Protons: Found in the nucleus. They have a positive charge.
Neutrons: Also found in the nucleus. They have no charge (they are neutral).
Electrons: Found in shells orbiting the nucleus. They have a negative charge.
The relative masses and charges of these particles are crucial facts to remember.
A critical point to grasp is that in any neutral atom, the number of positive protons in the nucleus is exactly equal to the number of negative electrons orbiting it. The +1 charge of a proton perfectly cancels out the −1 charge of an electron. This balance is the default state for an atom and is the foundation from which we can understand all chemical reactions. When this balance is disrupted, chemistry happens.
Atomic Number vs. Mass Number: The Atom's ID
How do we tell one element from another? The answer lies in the number of protons.
The Atomic Number, sometimes called the proton number, is the number of protons in the nucleus of an atom. This is the single most important number for an element. It is its unique identifier. An atom with 6 protons is always carbon. An atom with 8 protons is always oxygen. No exceptions. The periodic table is arranged in order of increasing atomic number.
The Mass Number is the total number of protons and neutrons in the nucleus. It tells us the mass of that particular atom. We don’t include electrons in the mass number because their mass is so tiny it’s considered negligible.
From these two numbers, we can figure out the complete composition of any atom.
Number of Protons = Atomic Number
Number of Electrons = Number of Protons (in a neutral atom)
Atomic Number + Number of Neutron = Mass number
Number of Neutrons = Mass Number – Atomic Number
Let’s look at an example. The element sodium is often represented like this:

The bottom number is the atomic number: 11. This tells us a sodium atom has 11 protons.
Because it’s a neutral atom, it must also have 11 electrons.
The top number is the mass number: 23.
To find the number of neutrons, we calculate: Mass Number – Atomic Number = 23−11=12. So, this sodium atom has 12 neutrons.


For any ion, you can always work out its composition:
Protons: Never changes. It’s the atomic number.
Neutrons: Never changes. It’s Mass Number – Atomic Number.
Electrons: For a positive ion, subtract the charge from the atomic number. For a negative ion, add the charge to the atomic number.
The fact that the number of protons for an element is fixed, but the number of neutrons can vary, leads us to our next important concept.
What are Isotopes? The Same, But Different
You might have noticed that the mass numbers on the periodic table are often decimals (e.g., Chlorine is 35.5), not whole numbers. This is because of isotopes.
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons.
Because they have the same number of protons, isotopes of an element have the same atomic number and identical chemical properties (as chemical reactions involve electrons, not neutrons). However, because they have different numbers of neutrons, they have different mass numbers.
Think of isotopes like different models of the same car. They are all the same brand and model (the element), but one might be the standard version while another has a heavier, upgraded engine (the extra neutrons). They are fundamentally the same car and drive in a similar way, but they have slightly different masses.

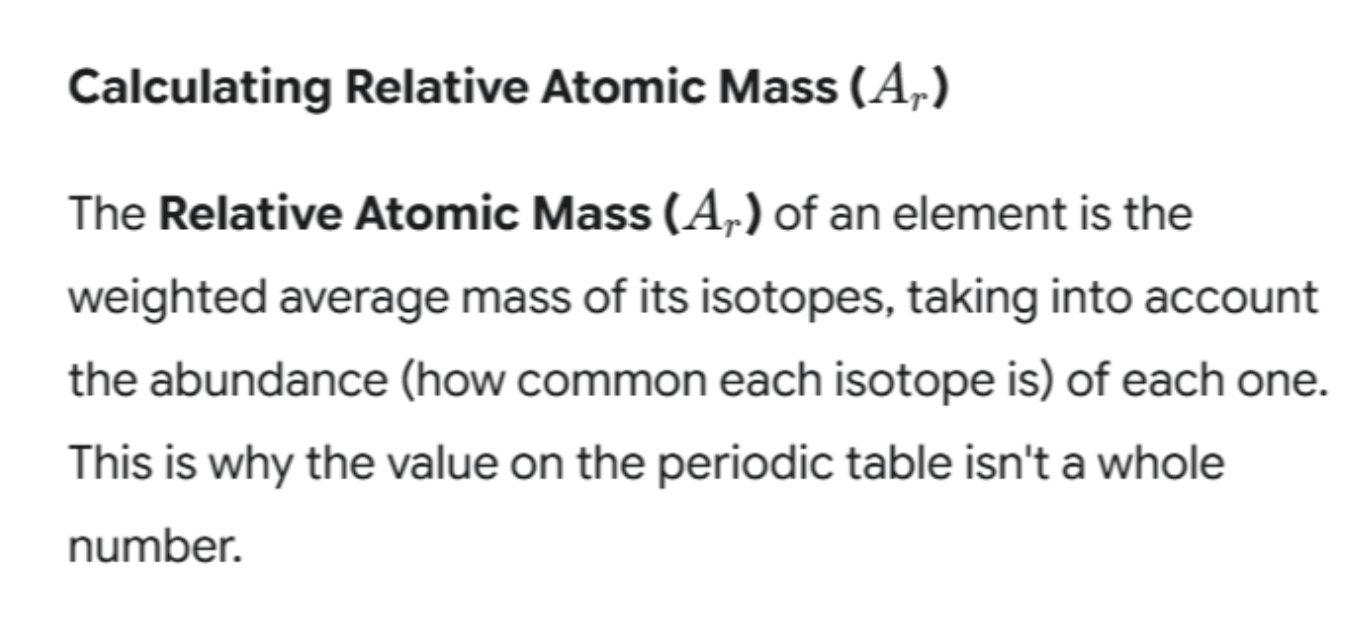
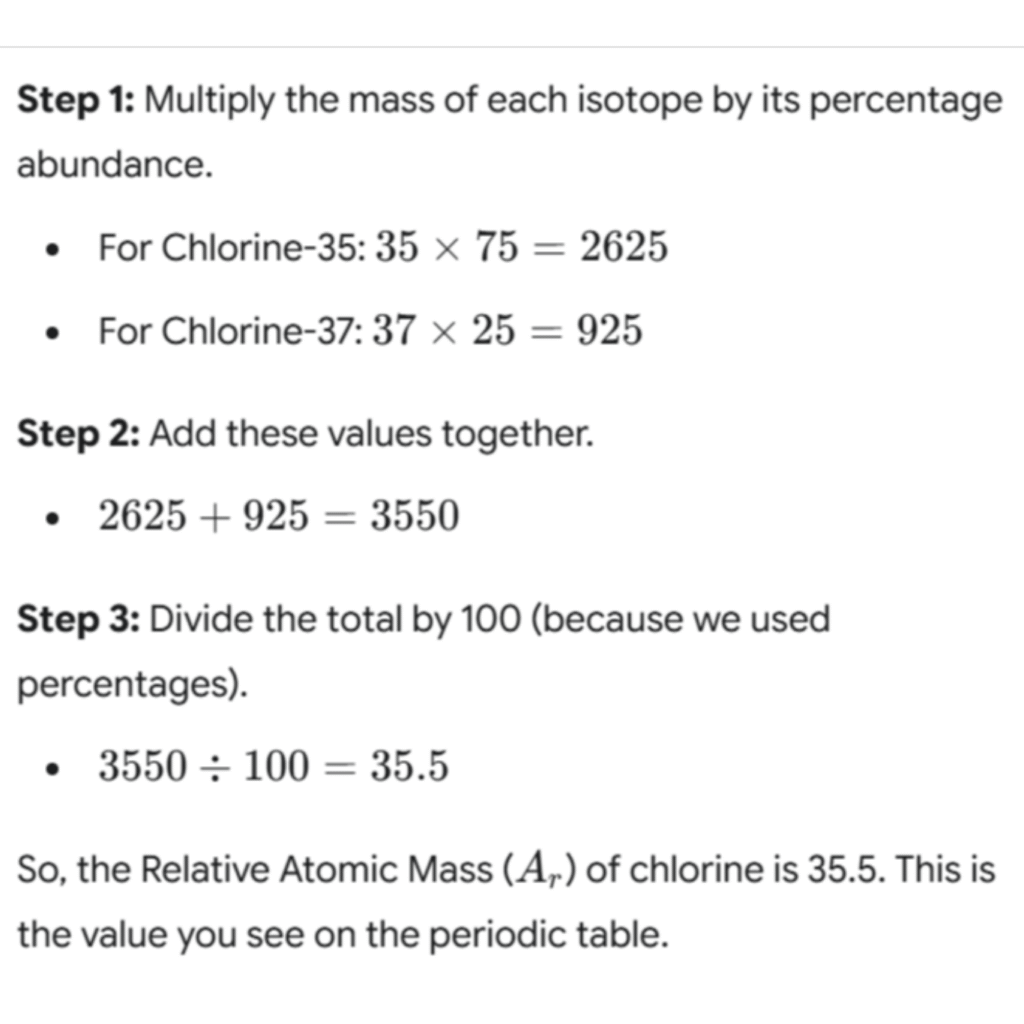
The calculation is a straightforward application of a weighted mean, a concept you may have encountered in maths. Here’s how to do it:
Worked Example: Calculating the Ar of Chlorine
In any sample of chlorine, you’ll find that roughly 75% of it is the Chlorine-35 isotope and 25% is the Chlorine-37 isotope.
Electron Shells and Configuration: The Rules of Arrangement
The electrons in an atom aren’t just flying around the nucleus randomly. They are arranged in specific energy levels called shells. Think of them like lanes on a running track around the nucleus. Each lane can only hold a certain number of runners.
For GCSE, you need to know the rules for the first 20 elements:
The 1st shell (closest to the nucleus) can hold a maximum of 2 electrons.
The 2nd shell can hold a maximum of 8 electrons.
The 3rd shell can also hold a maximum of 8 electrons.
Electrons always fill the lowest available energy level first, so they fill the shells from the inside out.
The way electrons are arranged is called the electronic structure or electron configuration. We can show this in two ways:
Numerically: We write the number of electrons in each shell, separated by commas. For example, sodium (Na) has 11 electrons. Its electronic structure is 2,8,1.
Diagrammatically: We draw a circle for the nucleus (writing the element’s symbol inside) and then draw concentric circles for the shells, using dots or crosses to represent the electrons.
This electronic structure is incredibly powerful because it is the direct link between an atom’s identity and its position on the periodic table. It is the code that explains everything about an element’s chemical behaviour.
The number of occupied shells tells you which period (row) the element is in. Sodium (2,8,1) has 3 occupied shells, so it’s in Period 3.
The number of electrons in the outermost shell tells you which group (column) the element is in. Sodium has 1 electron in its outer shell, so it’s in Group 1.
The electrons in the outermost shell are known as outer shell electrons or valence electrons. They are the most important electrons in the atom because they are the ones involved in chemical reactions.
Cracking the Code: The Periodic Table
The periodic table is the most important tool in chemistry. It’s not just a list of elements; it’s a map of their properties and relationships. But it didn’t always look the way it does today.
Early scientists tried to arrange the known elements in order of their atomic weights. This worked for some elements, but it led to problems where some elements were placed in groups with elements that had very different properties.
The breakthrough came from a Russian chemist named Dmitri Mendeleev in 1869. He also arranged elements by atomic weight, but he did two revolutionary things:
He left gaps in his table for elements he believed had not yet been discovered. He even used the properties of the elements around the gaps to predict the properties of these missing elements. When these elements were later discovered (like Gallium), their properties matched his predictions almost perfectly, which gave his table huge credibility.
He wasn’t afraid to swap the order of some elements if their properties meant they fitted better in a different group. He realised that the chemical properties were more important than a strict adherence to atomic weight order.
Mendeleev’s table was a work of genius, but it wasn’t perfect. The reason why ordering by atomic weight sometimes failed was only explained later with the discovery of isotopes. For example, Argon has a higher relative atomic mass than Potassium, but it is placed before it on the modern table.
The modern periodic table solves all these problems by arranging the elements in order of increasing atomic number (the number of protons). This arrangement means that elements with similar properties line up perfectly in vertical columns, called
groups. This is because, as we’ve just seen, elements in the same group have the same number of electrons in their outer shell, which gives them similar chemical properties. The table is called ‘periodic’ because these similar properties occur at regular intervals.
Metals vs. Non-Metals: A Great Divide
The periodic table has a clear dividing line running like a staircase from the top left to the bottom right.
Elements to the left of this line are metals.
Elements to the right of this line are non-metals.
The vast majority of elements are metals. They share a set of characteristic physical properties: they are typically strong, malleable (can be hammered into shape), good conductors of heat and electricity, and have high melting and boiling points.
Non-metals, on the other hand, are often dull, brittle (if solid), and poor conductors of heat and electricity (insulators).
This difference in physical properties is rooted in a fundamental difference in their atomic structure and chemical behaviour. The ultimate goal for an atom in a reaction is to achieve a full outer shell of electrons, like the noble gases. This is a very stable, low-energy state.
Metals generally have only a few electrons in their outer shell (e.g., 1, 2, or 3). It is much easier for them to lose these few electrons to achieve a full outer shell underneath. When they lose negative electrons, they form positive ions (cations).
Non-metals have outer shells that are more than half-full (e.g., 5, 6, or 7 electrons). It is much easier for them to gain the few electrons needed to complete their outer shell. When they gain negative electrons, they form negative ions (anions).
Let’s look at a classic metal you can see right here in Dubai: Aluminium (Al). It is in Group 3, with an electron configuration of 2,8,3. It readily loses its 3 outer electrons to form an Al3+ ion. Its properties of being strong, lightweight, and resistant to corrosion (it forms a tough, protective layer of aluminium oxide on its surface) make it the perfect material for the stunning facade of the Burj Khalifa, the world’s tallest building
Exploring the Groups: The Periodic Table Families
The vertical columns on the periodic table are called groups. Elements in the same group share similar chemical properties because they have the same number of electrons in their outer shell. We will now look at three key groups you need to know for your GCSE.
Group 0: The Noble Gases
The elements in Group 0 (sometimes called Group 8) are the Noble Gases: Helium (He), Neon (Ne), Argon (Ar), and so on.
Their defining feature is that they are extremely unreactive, or inert. They don’t readily form compounds with other elements. The reason for this lies in their electron configuration. They all have a full outer shell of electrons (Helium has 2, all the others have 8). This is an incredibly stable arrangement, so they have no tendency to lose, gain, or share electrons. They are happy as they are!
Because they are so stable, they exist as individual, single atoms (they are monatomic).
As you go down Group 0, the boiling points of the elements increase. This is because the atoms get larger, leading to stronger forces between the atoms which require more energy to overcome.
A fantastic example of a noble gas in action can be seen lighting up the Dubai skyline. Neon (Ne), when sealed in a glass tube with a high voltage passed through it, glows with a brilliant, iconic reddish-orange light. This property is used to create the vibrant, eye-catching advertising signs and artistic displays you see in malls, restaurants, and shops all across the city.
Group 1: The Alkali Metals
The elements in Group 1 are the Alkali Metals: Lithium (Li), Sodium (Na), Potassium (K), etc.
They are typical metals but have some unusual properties: they are very soft (you can cut them with a knife) and have low densities (Lithium, Sodium, and Potassium are less dense than water and will float on it).
Chemically, they are defined by the fact they all have a single electron in their outer shell. They are extremely keen to lose this one electron to form a positive ion with a +1 charge (e.g., Na+), giving them the stable electron structure of a noble gas. This makes them highly reactive.
The most important thing to understand about Group 1 is the trend in reactivity. As you go down the group, the alkali metals become more reactive. The reason for this is all about atomic structure:
Atomic Size: As you go down the group, each element has one more shell of electrons than the one above it. This means the atoms get bigger.
Distance: The single outer electron is further away from the positive pull of the nucleus.
Shielding: The inner shells of electrons “shield” the outer electron from the full attractive force of the nucleus.
Weaker Attraction: The combination of greater distance and more shielding means the electrostatic attraction between the positive nucleus and the negative outer electron gets weaker as you go down the group.
Easier to Lose: Because the attraction is weaker, less energy is needed to remove the outer electron. It is lost more easily, making the element more reactive.
So, Potassium is more reactive than Sodium, which is more reactive than Lithium.
You can see an application of Sodium (Na) every night in Dubai. The characteristic bright yellow-orange glow of many streetlights comes from high-pressure sodium-vapor lamps, which are highly efficient for lighting large areas.
Group 7: The Halogens
The elements in Group 7 are the Halogens: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), etc.

The trend in reactivity for the halogens is the opposite of the alkali metals. As you go down the group, the halogens become less reactive. The reasoning, however, is based on the exact same principles:
Atomic Size: As you go down the group, the atoms get bigger because they have more electron shells.
Distance: The outer shell is further away from the positive nucleus.
Shielding: There is more shielding from the inner electron shells.
Weaker Attraction: The attraction from the positive nucleus to capture an incoming electron into the outer shell becomes weaker.
Harder to Gain: Because the pull from the nucleus is weaker, it is harder for the atom to attract and gain the extra electron it needs. This makes the element less reactive.
So, Fluorine is the most reactive halogen, and reactivity decreases down to Iodine.
Chlorine (Cl) is a halogen with vital uses here in Dubai. Its ability to kill bacteria and other harmful microorganisms makes it an essential disinfectant. You’ll find it keeping the water safe in the countless swimming pools in apartments, villas, and hotels across the emirate. Furthermore, as the UAE relies on desalination to produce most of its fresh water from the sea, chlorination is a critical step in the process to purify the water and make it safe to drink.
Mastering these trends in reactivity for both Group 1 and Group 7 is key to exam success, as they are very common questions. The reasoning can feel tricky at first. If you find yourself needing a bit more guidance or want to tackle practice questions with an expert, getting help from one of our Dubai chemistry tutors can make all the difference.
Conclusion: You've Cracked the Code!
Congratulations! You have now covered all the core concepts of Atomic Structure and the Periodic Table for your AQA GCSE Combined Science exam. By understanding these fundamental principles, you have built the foundation for understanding almost everything else in chemistry.
Let’s quickly recap the absolute key takeaways:
An atom’s identity is its atomic number (the number of protons).
The periodic table is arranged by atomic number, grouping elements with similar properties.
An element’s group number tells you the number of outer shell electrons, which determines its chemical properties.
Chemical reactions are all about atoms trying to achieve a full outer shell of electrons, just like the unreactive Noble Gases.
Metals lose electrons to do this, while non-metals gain electrons.
Reactivity trends in Groups 1 and 7 can be fully explained by considering atomic size, shielding, and the nucleus’s attraction to the outer shell.
You now have the tools to look at the world around you from the architecture of Downtown Dubai to the water in your swimming pool and understand it on a much deeper, chemical level. Keep practising, reviewing these key ideas, and you will be well on your way to success in your exams.